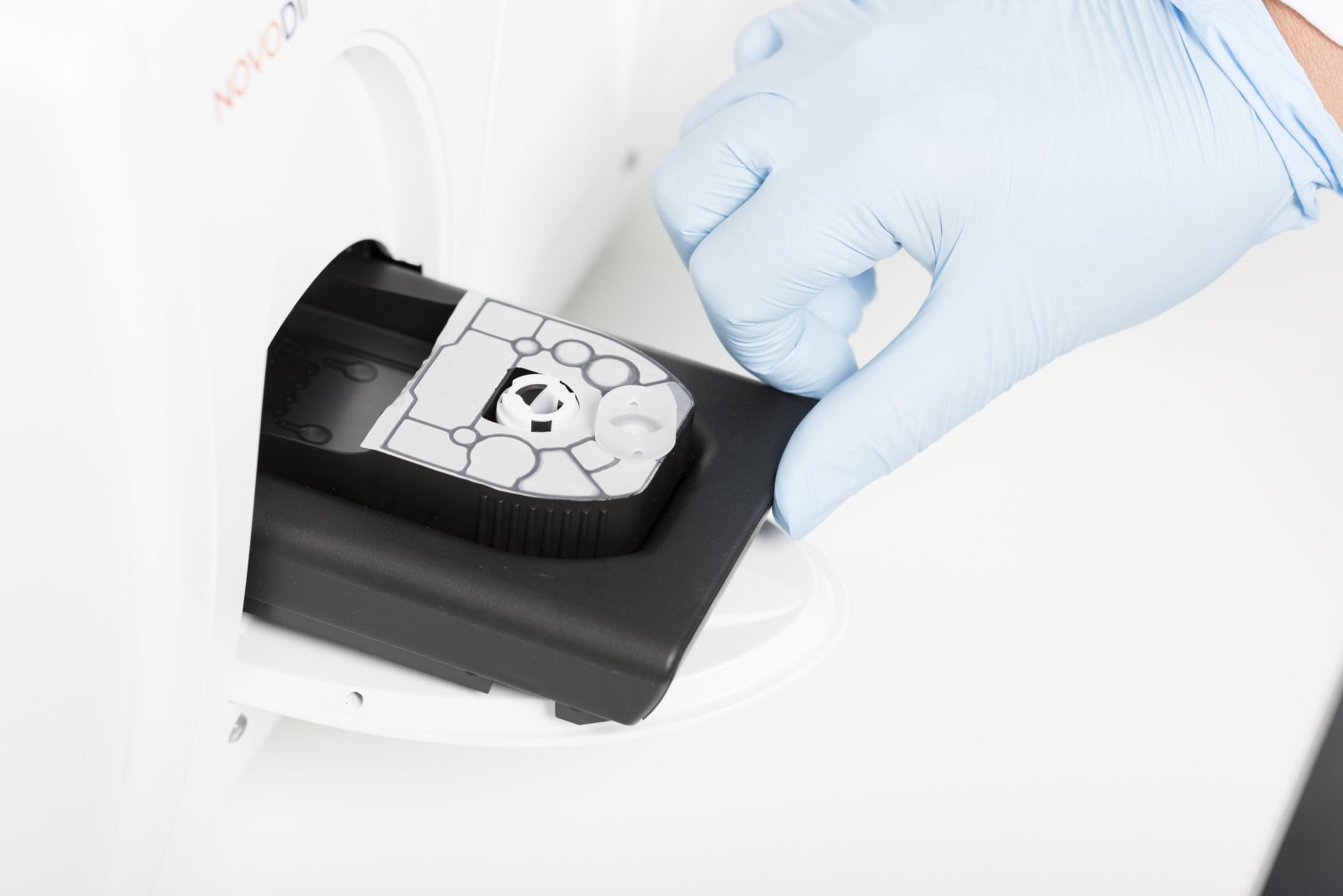
CE-IVD marking of Novodiag® CarbaR+, the 3rd molecular test on the automated system Novodiag®, for detection of the main Carbapenemase-Producing Enterobacteriaceae (CPE) and colistin resistance markers in one single test.
ESPOO, Finland, January 7th, 2019 – Mobidiag Ltd. today announces the CE-IVD marking of Novodiag® CarbaR+. Novodiag® CarbaR+ is a single molecular test allowing fully automated detection of Carbapenemase-Producing Enterobacteriaceae (CPE), and associated resistance markers. CPE are bacteria that are resistant to carbapenem class of antibiotics. In addition, Novodiag® CarbaR+ identifies a plasmid-mediated resistance to colistin. Colistin is considered the drug of last resort for many infections.